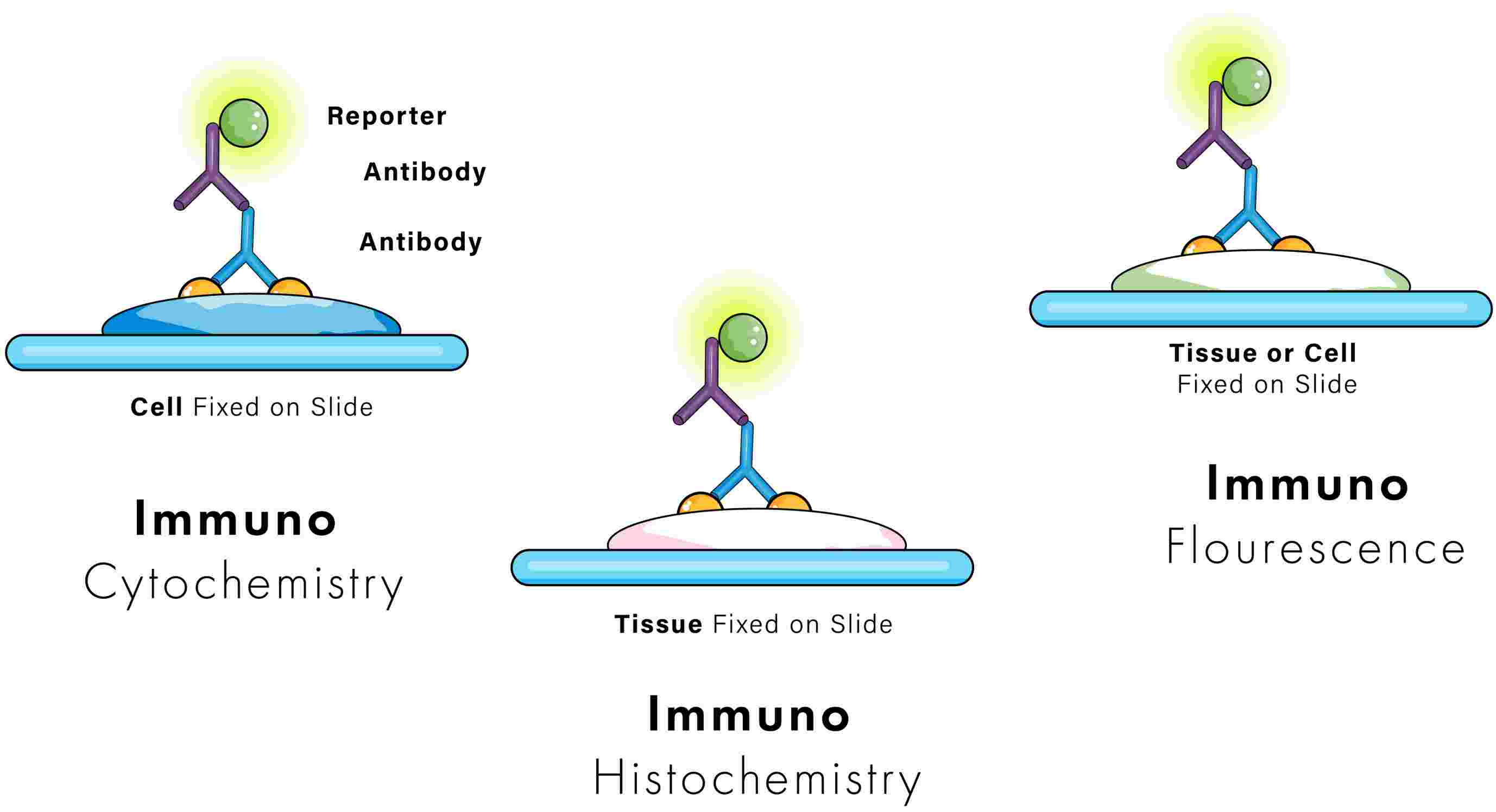
Immunohistochemistry (IHC) and immunofluorescence (IF) are two diagnostic methods that based on the concept of antigen and antibody binding, although the mode of detection in both techniques differ.
In IHC, enzymes and chromogens are used to produce colorful precipitates at the sites of antigen-antibody complexes. This is referred to as a chromogenic reaction. The colorful precipitates are usually brown or red, but can also be other colors. The colored chromogens are then visualized using a routine light microscope.
With IF, the primary antibody is often conjugated to a fluorophore, a fluorescent dye that fluoresces upon exposure to light of a specific wavelength. The fluorophore can be visualized using a fluorescent microscope and corresponding excitation filters. Common fluorophores include fluorescein isothiocyanate (FITC), auramine O, rhodamine B, acridine orange, and Texas red, although there are many others. Fluorophores are typically green, red, orange, and blue, but can be a variety of other colors.
The Strengths and Limitations of Immunohistochemistry
IHC scores with its long-lasting staining, compatibility with routine histological samples, and the ability to provide a permanent record of staining. As with any technique, IHC isn't flawless. Its main limitation is the inability to perform multicolor staining efficiently and lack of high-resolution imaging when compared to IF.
The Strengths and Limitations of Immunofluorescence
IF offers several advantages, including multicolor imaging and live cell imaging capabilities. But what steals the show is its high resolution, capturing breathtaking images of cellular life. While IF offers significant benefits, it is not without limitations. The need for specialized expensive equipment for fluorescence microscopy could be a stumbling block for some labs. In addition, fluorophores can fade over time, a phenomenon known as photobleaching. This means that they need to be reviewed rapidly and stored appropriately before degradation occurs.
| IHC | IF | |
| Sample type | FFPE or fresh/frozen | FFPE or fresh/frozen |
| Molecule functioning as the biological tag | Primary antibody | Primary antibody |
| Method used to visualize the antigen-antibody complex | Enzymes and chromogens | Fluorophores |
| Type of microscope needed to examine the tissues | Light microscope | Fluorescence microscope with compatible excitation and barrier filters |
| Resolution | Detail and depth of interrogation are limited by the quality and thickness of the samples. | Maximum detail can be detected using confocal microscopy, including compilation of 3D images using scanning laser techniques to visualize within much thicker sample slices. |
| Color stability | Stains are stable and do not fade during examination under the microscope. Slides can be stored for years. | Photostability can be variable across fluorescent conjugates, making data collection time sensitive. Permanent mounting media can increase longevity of archived slides. |
| Compatible with multiplexing? | Very limited, a maximum of 2 to 3 chromogens per slide | Yes, limited only to the number of fluorophores available and the capability of the digital pathology software. |
| Common artifacts | Non-specific background staining due to endogenous enzymes | Autofluorescence, fading of fluorophores over time |
Considerations for Choosing a Chromogenic or Fluorescent Detection Method
How abundant is your protein of interest?
In general, chromogenic techniques will provide higher sensitivity when paired with appropriate signal amplification protocols, including incubation with either avidin-biotin complex (ABC) or labeled streptavidin-biotin (LSAB), both of which can increase signal several-fold over a labeled secondary antibody.
Do you need to label more than two targets?
Due to the limited selection of chromogenic reagents, fluorescent markers are superior for detecting multiple signals from the same tissue sample. Fluorescent markers can be manipulated by changing the excitation wavelength and intensity, and spectra can be analyzed for potential overlap prior to staining. Overlapping chromogenic staining, in contrast, can yield confusing or misleading results.
Do you need to identify any co-localized targets?
Fluorescent markers are more suitable for detecting the colocalization of two or more target proteins in the same cell structures. Fluorescent markers can be analyzed independently, while chromogenic staining will either blend or overlap. Overlaying multiple fluorescent images from the same tissue sample can provide a complete picture of protein expression and interactions.
Do you need to preserve samples for an extended time?
Under normal conditions, fluorescent labels are more prone to photobleaching, which will result in a diminished signal over time. Since the chromogenic reaction relies on the substrate changing from liquid to solid state, the labeling is more durable.